Increased mercury emissions from modern dental amalgams
Link: /uploads/arquivos/Increased_mercury_emissions_from_modern_dental_ama.pdf
Ulf G. Bengtsson. Lars D. Hylander
Abstract
All types of dental amalgams containmercury, which partly is emitted as mercury vapor.
All types of dental amalgams corrode after beingplaced in the oral cavity. Modern high copperamalgams exhibit two new traits of increased insta-bility.
Firstly, when subjected to wear/polishing,droplets rich in mercury are formed on the surface,showing that mercury is not being strongly bonded tothe base or alloy metals.
Secondly, high copper amalgams emit substantially larger amounts of mer-cury vapor than the low copper amalgams used beforethe 1970s.
High copper amalgams has been devel-oped with focus on mechanical strength and corrosionresistance, but has been sub-optimized in otheraspects, resulting in increased instability and higheremission of mercury vapor.
This has not beenpresented to policy makers and scientists. Both lowand high copper amalgams undergo a transformationprocess for several years after placement, resulting ina substantial reduction in mercury content, but there exist no limit for maximum allowed emission ofmercury from dental amalgams.
These modern highcopper amalgams are nowadays totally dominatingthe European, US and other markets, resulting insignificant emissions of mercury, not consideredwhen judging their suitability for dental restoration.
Keywords: Mercury · Non-gamma-two ·Non-ɣ2 · Copper amalgam
OBS: Polishing the surface of high copper amalgam fillings stimulates the formation of droplets rich in mercury. This formation happens even if the polishing takes place under cold water to avoid any rise in temperature & continues a number of hours after the polishing has stopped.

Two tablets of copper amalgam in a spoon heated over an open flame ready to be crushed.
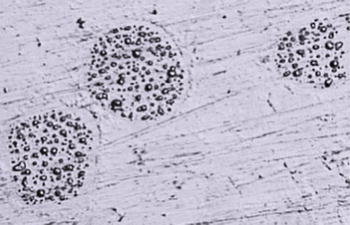
Droplets of mercury on the surface of modern, high copper non-ɣ2-amalgam, photographed with a light microscope (×252 magnifying).
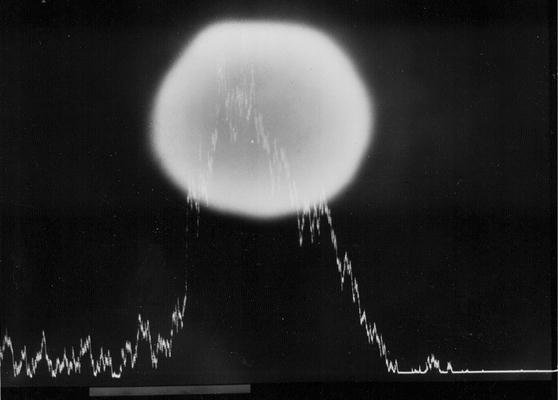
A sphere of mercury on the surface of modern, high copper non-ɣ2-amalgam, documented with a scanning electron microscope (SEM). Note the strong signal from mercury as the electron beam passes the sphere.
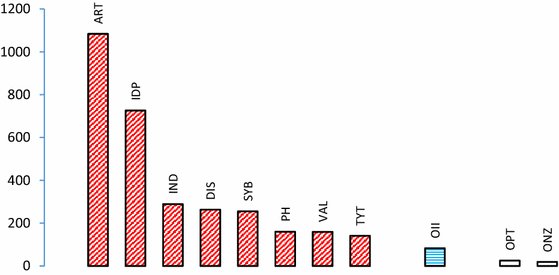
Mercury vapour loss (ng) between 0.5 and 30 min after abrasion. Left group (red cross-hatched bars): non-ɣ2-amalgams; third bar from right (blue hatched): reduced ɣ2-amalgam; right group (two white bars): old, conventional ɣ2–containing amalgams Diagram based on findings in Mahler et al. (1994).
