XPS, AES and SEM analysis of recent dental implants
Link: http://citeseerx.ist.psu.edu/viewdoc/download;jsessionid=66F26A7E885A181E807EFA23B04234F8?doi=10.1.1.610.507&rep=rep1&type=pdf
Byung-Soo Kang a,*, Young-Taeg Sul a , Se-Jung Oh b , Hyun-Ju Lee c , Tomas Albrektsson a a Department of Biomaterials/Handicap Research, Institute for Clinical Sciences, The Sahlgrenska Academy at Gothenburg University, Box 412, SE 405 30, Gothenburg, Sweden bDepartment of Physics and Astronomy, Seoul National University, Seoul, South Korea c National Center for Inter-University Research Facility, Seoul National University, Seoul, South Korea
Abstract
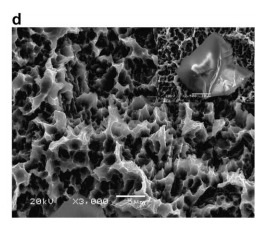
Today, surface chemistry modifications of titanium implants have become a development strategy for dental implants. The present study investigated the chemistry and morphology of commercially available dental implants (Nobel biocare TiUnite, Astra AB OsseoSpeed, 3i Osseotite, ITI-SLA). X-ray photoelectron spectroscopy (XPS) and auger electron spectroscopy were employed for the analysis of surface chemistry. The morphology was investigated by scanning electron microscopy. The present study demonstrated the major differences of surface properties, mainly dependent on the surface treatment used. The blasting and acid etching technique for the OsseoSpeed, Osseotite and SLA surfaces generally showed mainly TiO2, but a varying surface morphology. In contrast, the electrochemical oxidation process for TiUnite implants not only produces microporous surface (pore size: 0.5–3.0 lm), but also changes surface chemistry due to incorporation of anions of the used electrolyte. As a result, TiUnite implants contain more than 7 at.% of P in oxide layer and higher amounts of hydroxides compared to the other implants in XPS analysis. F in OsseoSpeed implants was detected at 0.3% before as well as after sputter cleaning.
Keywords: Clinical dental implants; Surface chemistry; Morphology; X-ray photoelectron spectroscopy (XPS); Titanium