Systemic and Local Tissue Response to Titanium Corrosion
Link: http://cdn.intechopen.com/pdfs/33623/InTech-Systemic_and_local_tissue_response_to_titanium_corrosion.pdf
Authors:
Daniel Olmedo,
Deborah Tasat,
Gustavo Duffó,
Rómulo Cabrini,
María Guglielmotti,
University of Buenos Aires
National Research Council (CONICET)
National University of General San Martin
National Atomic Energy Commission (Argentina)
1. Introduction
The term biomaterials refers to materials that have been designed to be implanted or placed inside a live system with the aims to substitute or regenerate tissue and tissue functions. Williams defines biomaterials as those that are used in devices for biomedical use designed to interact with biological systems (Williams, 1986). Classically, biomaterials are divided into four types: polymers, metals, ceramics and natural materials. Two different types of biomaterials can be combined to obtain a fifth type known as composite biomaterials (Abramson et al., 2004). Biomaterials are widely used in orthopedic, dental, cardiovascular, ophthalmological, and reconstructive surgery, among other applications. The discovery of relatively inert metals and alloys has led to their use in the field of biomedical applications such as orthopedics and dentistry, and their use in increasing due to their physical-chemical properties and compatibility with biological surroundings (Ratner et al., 2004). One of the most frequently employed metallic biomaterials is titanium (Anderson et al., 2004). Though zirconium is not widely used as a clinical material, it is chemically closely related to and has several properties in common with titanium (Thomsen et al., 1997). Although both titanium and zirconium are transition metals, their physicochemical properties such as oxidation velocity, interaction with water, crystalline structure, transport properties, and those of their oxides differ quantitatively (Henrich & Cox, 1994); these differences may have an effect on biological response (Thomsen et al., 1997). Indeed, the use of zirconium and zirconium alloys to manufacture implants for traumatological, orthopedic, and dental applications has been reported (Sherepo et al., 2004; Sollazzo et al., 2007).
Titanium and zirconium are highly reactive metals and when exposed to fluid media or air, they quickly develop a layer of titanium dioxide (TiO2) or zirconium dioxide (ZrO2). This layer of dioxide forms a boundary at the interface between the biological medium and the metal structure. It produces passivation of the metal, determining the degree of biocompatibility and the biological response to the implant (Kasemo 1983, Kasemo & Lausmaa 1988, Long & Rack, 1998). Titanium dioxide exists naturally, mainly in the form of three crystalline structures: rutile, anatasa, and brookite. In the case of titanium implants, the passive oxide layer is composed of anatase and rutile or anatase alone (Effah et al., 1995; Olmedo et al., 2008a; Sul et al., 2001). Zirconium, however, does not exist as a free metal in nature; it occurs as the minerals zircon, or zirconium silicate (ZrSiO4), and the rare mineral baddeleyite or zirconium dioxide (ZrO2) which has a monoclinic crystal structure (Zirconium. Mineral Information Institute, 2009). Baddeleyite, also known as zirconia, is the most naturally occurring form and can be transformed into a tetragonal (1100 ºC) or cubic (2370 ºC) crystallographic form depending on temperature (Chowdhury et al., 2007; Manicone et al., 2007).
Titanium is widely used in the manufacture of dental and orthopedic implants due to its excellent biocompatibility. The latter is defined as the ability of a material to perform with an appropriate host response in a specific application (Williams, 1987). The use of titanium dental implants has revolutionized oral implantology. Currently, almost 300,000 patients in the United States have dental implants. In the area of orthopedics, replacement hip joints are implanted in more than 200,000 humans each year (Ratner et al., 2004). Dental implants are surgically inserted into the jaw bone primarily as a prosthetic foundation. The process of integration of titanium with bone was termed “osseointegration” by Brånemark (Brånemark et al., 1977; Chaturvedi, 2009).
No metal or metal alloy is completely inert in vivo. Corrosion is the deterioration of a metal due to interaction (electrochemical attack) with its environment, which results in the release of ions into the surrounding microenvironment (Jacobs, 1998). There are “noble” metals such as rhodium (Rd), palladium (Pd), iridium (Ir) and platinum (Pt), whose resistance to corrosion is due to their high thermodynamic stability. Passivating metals, such as titanium (Ti), vanadium (V), zirconium (Zr), niobium (Nb), and tantalum (Ta), however, are thermodynamically unstable and their resistance to corrosion results from the formation of a protective oxide layer on their surface (Lucas et al, 1992). Titanium is available as commercially pure (c.p.) titanium or as Ti-6Al-4V alloy with 6% aluminum and 4% vanadium. The addition of Al and V increases strength and fatigue resistance; however, this may affect the corrosion resistance properties and may result in the release of metal ions (Textor et al., 2001). C.p. titanium and Ti-6Al-4V alloy are the two most common titaniumbased implant biomaterials (Abramson et al., 2004). There are four standard types or grades of c.p. titanium used for the manufacture of surgical implants, which differ in their content of interstitial elements. This content determines the mechanical properties of a material: the higher the content the higher the grade. In other words, grade 1 is the most pure and grade 4 contains the greatest amount of impurities and has the greatest mechanical resistance. C.p. titanium is used to manufacture dental implants, whereas a Ti6Al4V alloy is used mostly in orthopedics.
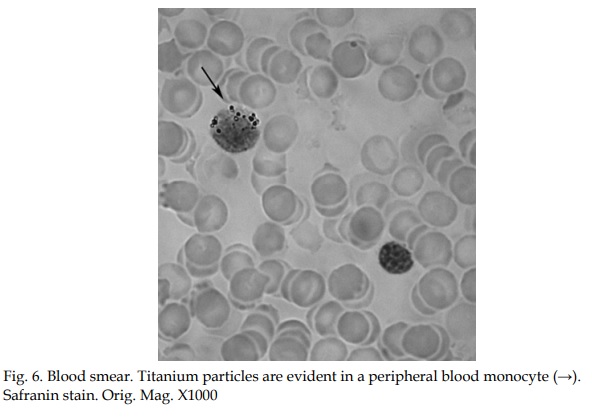
As previously stated, all the metallic materials employed in surgery as permanent implants are liable, to a certain degree, to corrosion due to variations in the internal electrolyte milieu (Jacobs, 1998). Corrosion, one of the possible causes of implant failure, implies the dissolution of the protective oxide layer. When metal particles/ions are released from the implant surface, they can migrate systemically, remain in the intercellular spaces near the site where they were released, or be taken up by macrophages (Olmedo 2003, 2008b). The presence of metallic particles in peri-implant tissues may not only be due to a process of electrochemical corrosion but also to frictional wear, or a synergistic combination of the two.
Moreover, mechanical disruption during insertion, abutment connection, or removal of failing implants has been suggested as a possible cause of the release of particles from metal structures (Flatebø, 2006; Jacobs, 1998). The release of particles/ions from the implant into the surrounding biological compartment, their biodistribution in the body, and their final destination are issues that lie at the center of studies on biocompatibility and biokinetics. The chemical forms of these released elements have not been identified to date. It is unclear whether these products remain as metal ions or metal oxides, or whether they form protein or cell-bound complexes (Brown et al., 1987; Urban et al., 2000). In the particular case of titanium, little is known about the valence with which it exerts its action, the organic or inorganic nature of its ligands, and its potential toxicity (Jacobs, 1991).
The potential toxicity and biological risks associated with ions and/or particles released due to corrosion of metallic implants is a public health concern for the community of patients who have a prosthesis (orthopedic and/or dental), since these prostheses remain inside the body over long periods of time. Likewise, the subject of corrosion is of interest to researchers; corrosion studies aim at avoiding the possible corrosion-related health problems that may arise when metallic implants are placed in humans. Controlling corrosion is most relevant for, in order to protect patient health, corrosion should be negligible. Thus, managing and controlling corrosion of a biomedical implant is a paramount issue from a biological, sanitary, metallurgic, economic and social point of view. The current massive use of these metal biomaterials in the biomedical field renders it necessary to have detailed knowledge not only on their early effects (short term failure) but especially on their long term effects, given that these materials remain inside the patients over long periods of time, sometimes throughout their entire life. With the aims to improve biocompatibility and mechanical resistance, manufacturers of biomedical implants seek to develop an adequate design with minimal degradation, corrosion, dissolution, deformation, and fracture.
The study of corrosion requires an interdisciplinary approach including chemists, biologists, physicists, engineers, metallurgists, and specialists in biomedicine. The Biomaterials Laboratory of the Department of Oral Pathology of the University of Buenos Aires, the National Commission of Atomic Energy and the University of San Martin have been conducting collaborative research on corrosion aimed at evaluating both local tissue response in the peri-implant microenvironment and the systemic effects and possible consequences of corrosion, focusing mainly on dental implants (Olmedo et al., 2009).